What are Crystals and How do They Form?
What is a Crystal?
The geological definition of a crystal is a naturally occurring inorganic solid made up of atoms arranged in a repeating three-dimensional pattern.
This internal arrangement is known as a crystal lattice. The structure forms as atoms or molecules organise themselves into a highly ordered arrangement during crystallisation.
When atoms or ions bond under the right conditions, they form a repeating structure. As this structure grows, it becomes a crystal.
The Word 'Crystals' Causes So Much Confusion
In recent years, the word crystals has been adopted to describe rocks and minerals used for their metaphysical healing properties.
This has caused so much confusion when discussing crystals in the geological sense.
In alternative therapies, a crystal is any naturally occurring object believed to have healing or spiritual properties. In some articles, even man-made materials like opalite and goldstone are described as crystals.
This overlap in terminology has made it particularly difficult to explain the difference between crystals, rocks, and minerals.
It’s not known exactly when the word crystals started being used in this way. What is known is that many ancient cultures, including the Egyptians, Greeks, and Chinese, used certain stones for protection, health, or spiritual rituals. However, they wouldn't have referred to them as crystals.
The modern use of this word likely gained popularity during the New Age movement in the 20th century, when interest in energy, chakras, and alternative healing focused on the natural beauty and symmetry of geological crystals.
Over time, it became a catch-all word for a wide variety of stones used for metaphysical purposes.
How Small is an Atom?
Atoms are the basic building blocks of everything in the universe, but they’re far too small to be seen, even with most microscopes.
Using AI, I asked how many atoms would fit on the head of a pin. This was the response:
A typical pinhead is about 1.5 millimetres across. The diameter of an atom is roughly 0.1 nanometres, that’s 0.0000000001 metres. Based on those sizes, around 4,216,000,000,000,000,000,000 atoms would fit on a pinhead.
If you're struggling to read that number, it's 42 quintillion, 160 quadrillion, which gives you a sense of just how small atoms are, and how precise and ordered they must be to form a crystal.
According to the late Professor Stephen Hawking, approximately fourteen billion years ago, the entire universe was the size of a single atom.
How Do Crystals Form?
This diagram shows an example of an orderly, repeating arrangement of atoms inside a crystalline solid.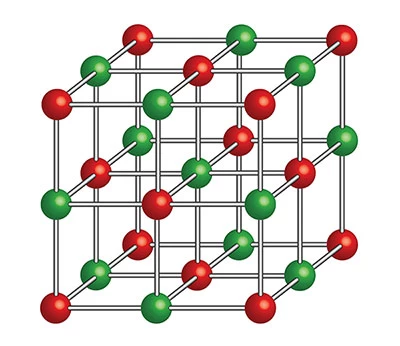
Crystals often form when liquids cool and solidify. The slower the cooling process, the more time they have to grow and the larger they become.
As a liquid cools and solidifies, its particles arrange themselves in an ordered, repeating pattern. This process of crystal formation, called crystallisation, gives a crystal its internal structure.
Crystals can also form through precipitation. Water can only hold a certain amount of dissolved minerals and salts. When that limit is reached, they can no longer remain dissolved.
At that point, the excess particles come together to form solid crystals. This process, where dissolved particles come out of a solution to form a solid, is called precipitation.
Obsidian, a type of volcanic glass, is a non-crystalline solid. This is because the molten lava from which obsidian is produced cooled so fast that there was no time for crystals to grow.
Granite, which is a rock, not a mineral, often contains large crystals. This is because the magma from which it formed cooled very slowly over millions of years.
Crystals have smooth surfaces known as faces and straight edges. While some, like quartz, are large enough to be seen with the naked eye, others are microscopic.
Some crystals are so small, they're difficult to see even with strong magnification.
Regardless of size, one thing that remains unchanged is that crystals of the same mineral variety always have the same crystal structure. If the structure changes, a different mineral will form.

The smallest piece of quartz is made up of quadrillions of atoms arranged in a crystal lattice. As the atoms come together in an orderly repeating arrangement, they form a crystalline solid.
An atom is the smallest unit of matter that makes up a chemical element.
When the arrangement of atoms within a naturally occurring solid does not form an orderly repeating pattern, it's not crystalline. These materials, which lack any significant crystal structure, are known as mineraloids.
A naturally occurring solid that lacks a crystalline structure cannot be classified as a mineral. By definition, minerals are crystalline.
What is Crystal Habit?
The repeating, three-dimensional arrangement of atoms inside a crystal influences its external shape. The term crystal habit refers to the natural shape of a crystal.
There are seven different crystal systems, and every mineral falls into one of these groups. All crystals show symmetry because they’re built from repeating geometric patterns.
Cube-shaped crystals, part of the isometric crystal system, are among the simplest and most common shapes.
One of the most recognisable shapes is hexagonal, often seen in quartz. These six-sided, elongated crystals typically end in a natural termination.
Common Examples of Crystals
Some of the most common examples of crystals include:
Quartz - made up of silicon and oxygen atoms
Halite - better known as table salt, a crystal form of sodium chloride
Calcite - made of calcium carbonate; often forms clear or white crystals
Amethyst - a purple variety of quartz, coloured by trace amounts of iron
Snow - crystallised water (ice), which is crystalline but not classified as a mineral in geology
Frequently Asked Questions
What is the geological definition of a crystal?A crystal is a naturally occurring solid made up of atoms arranged in a highly ordered, repeating three-dimensional pattern called a crystal lattice.
Are healing crystals actual crystals?
Sometimes. The word 'crystals' is a generic term for any material believed to have healing properties. Many are minerals, like quartz or amethyst, but others, such as shungite or obsidian, are not minerals and do not have a crystalline structure, so they're not crystals in the geological sense.
How do crystals form?
Crystals usually form when liquids cool and solidify. As they cool, their particles arrange in an ordered, repeating pattern. They can also form when dissolved minerals precipitate out of water.
Why are some crystals microscopic and others large?
Crystal size depends on how quickly a liquid cools. Slow cooling allows crystals to grow larger; rapid cooling results in small or microscopic crystals.
Are all naturally occurring solids crystals?
No. A naturally occurring solid must have an orderly, repeating arrangement of atoms to be crystalline. Solids without this structure are non-crystalline. Obsidian is one example. These materials are correctly known as mineraloids.
What’s the difference between a crystal, a rock, and a mineral?
A crystal is made up of atoms arranged in a repeating pattern. A mineral is a naturally occurring, inorganic solid with an internal crystalline structure. A rock is composed of one or more minerals.
Article Pictures
Our first picture, a cluster of quartz crystals, and the final picture, gold crystals, are courtesy of Stan Celestian.
The blue, cube-shaped fluorite crystals on quartz is courtesy of Ron Wolf.










